

Early Access Programs
Design, implementation and global management of Expanded Access, Early Access, Compassionate Use and Named Patient Programs


Design, implementation and global management of Expanded Access, Early Access, Compassionate Use and Named Patient Programs
Upcoming Webinar
Choose your session and secure your spot:
Upcoming Webinar
Choose your session and secure your spot:
We partner with pharma and biotech organizations to deliver customized programs that transform lives. These partnerships have made us a leader in the provision of Expanded Access, Early Access, Compassionate Use and Named Patient Programs across 98 countries.

Over the last 20 years, we’ve gained the experience it takes to strategically advise, design, initiate and manage global or regional programs with you.
End-to-end solutions
We provide end-to-end solutions to manage complex EAPs. Whatever your size and scale, we’re dedicated to empowering patient access to the right therapy.
Why partner with Smartway?
An Early Access Program (EAP) gives access to innovative therapies that are not available in the country of treatment.
EAP’s are known by differing names depending on the regulatory jurisdiction. These include Expanded Access, Early Access, Compassionate Use and Named Patient Programs.

EAPs are complicated. They are affected by numerous variables – not just regulatory jurisdictions, but also how they link to asset development and commercialization plans.
Our consultancy team can help you decide if an EAP is right for your asset, find the optimal timing, understand the regulatory structure, and integrate your EAP with RWD and commercialisation.

At Smartway, we see regulatory strategy and program design as being totally integrated. Our regulatory team comes with deep knowledge of the various regulatory schemes and jurisdictions around the world.
We deliver programs focussed on your objectives, focussed on delivering success now, not trying to introduce other services or partner companies.

EAPs are designed to allow patient access to new therapies, but they can also provide an opportunity to collect Real World Data (RWD) and generate Real World Evidence (RWE).
We’ll work with you to evaluate the correct level of RWD collection for your program, building this into our compliant program design. We collect the data in our 21CFR Part 11 compliant management system SMARThub®
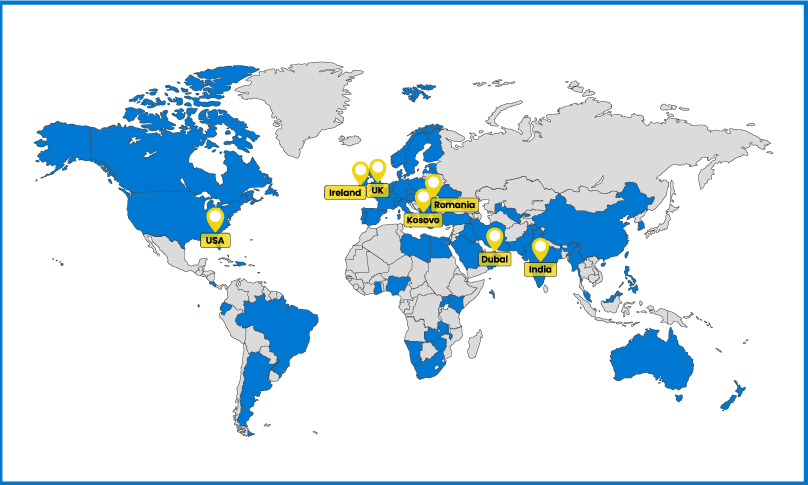
Smartway distributes pre-approval therapies to over 98 countries across the globe. In the last 5 years, we’ve shipped over 5 million of medication worldwide.
We use our fully owned and controlled strategic supply hubs in the EU, UK, UAE and India to provide quick, accurate supply, everywhere.

Your EAP project will be led by dedicated project managers while also bringing in expertise from across Smartway.
SMARThub® - Supporting Medicines Access in Real Time Hub, our bespoke management system. 21CFR Part 11 compliant the online system provides an easy way for Physicians, Pharmacists and Hospitals to register to the program, order supplies and be provided with all relevant information.

We unapologetically put the patient at the heart of every program. We understand your objectives and deliver on them, but we never lose sight of the patient and the impact these programs can have on them and their families.

EAP is a rapidly changing area. The Smartway team is constantly conducting regulatory horizon scanning and conducting global market research. We then analyse the information and changes and produce concise updates and briefings focused on the EAP market. We also run expert webinars and interactive training. For the latest news and articles please visit our resource centre.