In recent months, patients across the UK who are affected by pancreatic insufficiency, a condition that severely affects digestion and overall health, have faced real personal challenges and distress due to a nationwide shortage of Creon. In response to this crisis, Smartway is sourcing, importing and supplying licensed Canadian Creon, helping to ensure continuity of care. By working closely with manufacturers, suppliers, healthcare professionals and patients, we aim to contribute to maintaining access to the life-saving treatment that patients rely on.
Understanding the Importance of Creon
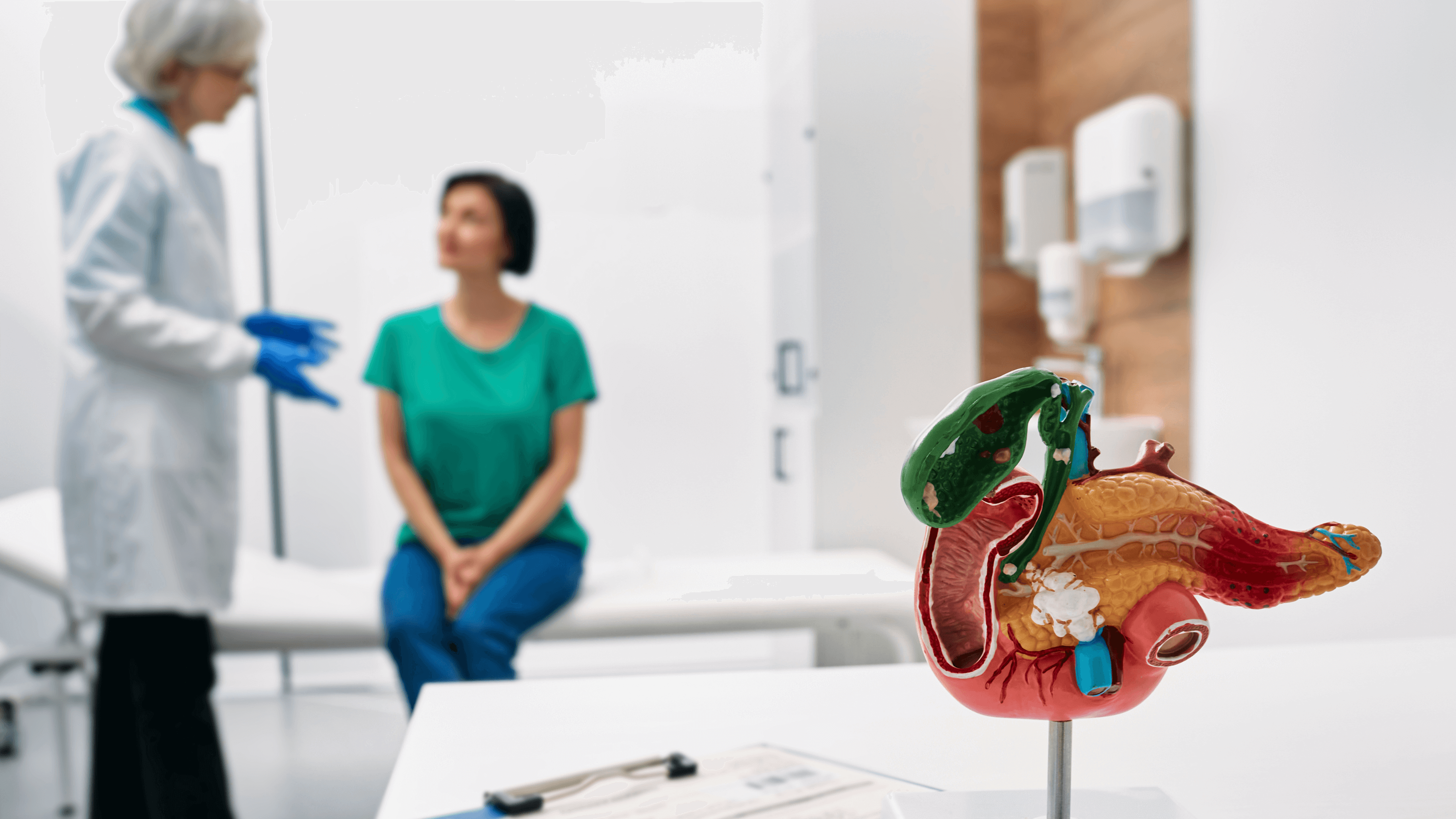
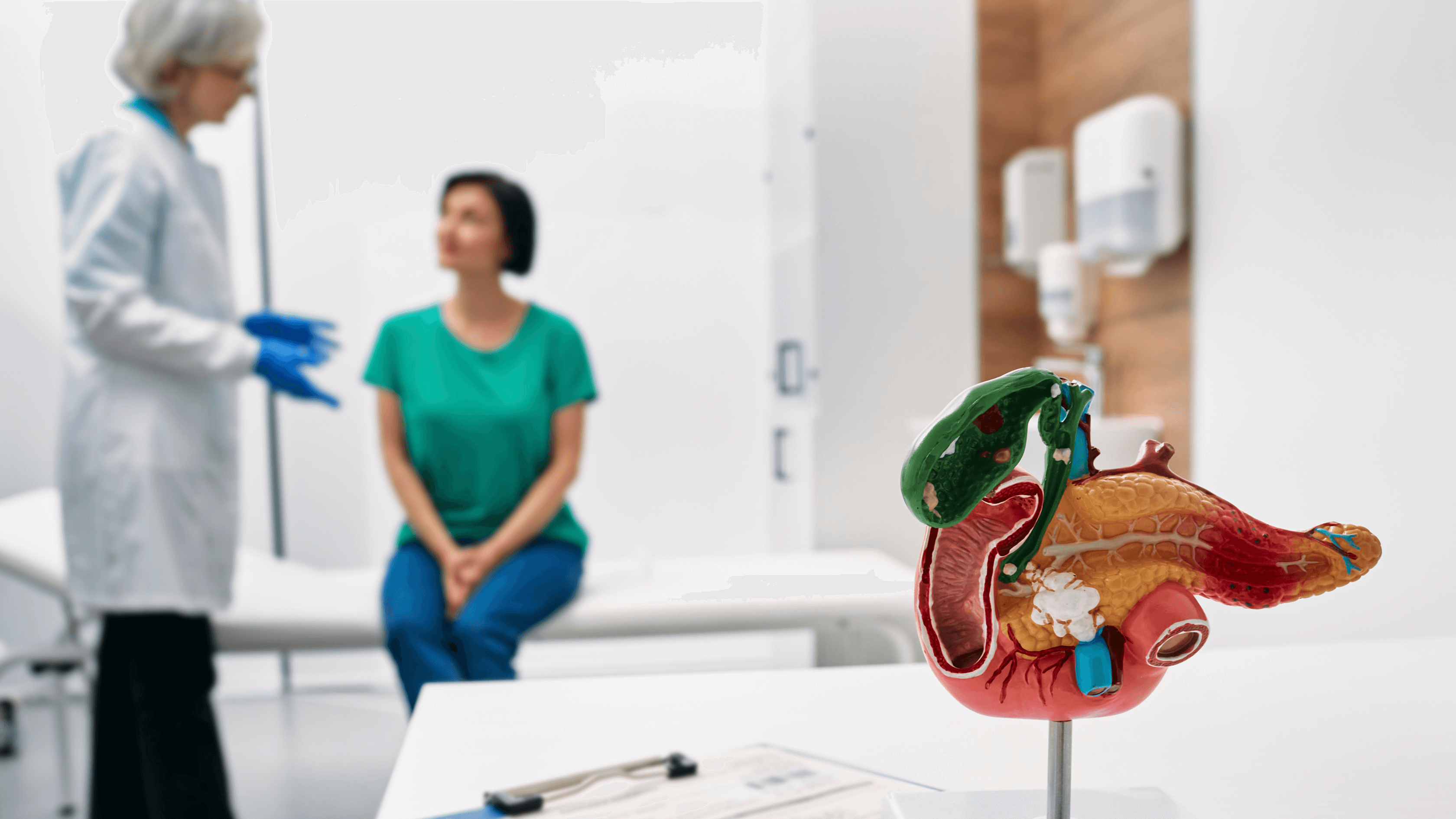
Creon capsules are a form of pancreatic enzyme replacement therapy (PERT) used to support digestion in individuals with conditions such as cystic fibrosis, chronic pancreatitis, and pancreatic cancer. These capsules contain essential enzymes - lipase, amylase, and protease - that break down fats, carbohydrates, and proteins, enabling the body to absorb nutrients properly. Access to Creon is critical for many patients to maintain their quality of life and address their symptoms.
The Nature of the Shortage
Manufacturing issues, supply and distribution challenges, government interventions, and drug recalls are just a few reasons medicine shortages occur. Addressing these shortages, where domestic manufacturing capabilities cannot, requires a combination of an extensive supply network and effective procedures to ensure access to medicines, which improves patient safety.
The current shortage in the UK has been attributed to several factors, including manufacturing delays, increased demand, and supply chain disruptions exacerbated by global events. Reports indicate that some patients have been unable to find their prescribed dosage, leading to health complications and heightened anxiety. While the National Health Service (NHS) and healthcare providers have been working to manage the shortage, the gap in supply has created urgent needs that must be addressed by looking beyond the UK.
For decades, Smartway have been committed to consistently providing safe and effective licensed and unlicensed medicines to pharmacies, as well as NHS and private hospitals. Our global procurement network, built over the past 30 years, is designed to be resilient to the issues that cause shortages. This resilience enables us to deliver medicines safely and promptly, wherever and whenever patients need them. We establish unique processes and arrangements with customers, suppliers, manufacturers, and biotech companies to address those urgent needs.
The Canadian Solution
In response to the Creon shortage, the UK government and healthcare authorities have explored alternative solutions. One such option is the importation of licensed Creon from Canada. Canadian Creon is manufactured under strict regulatory guidelines, ensuring it meets safety and efficacy standards similar to those in the UK.
Importing licensed Canadian Creon helps bridge the gap caused by the shortage and provides patients with a reliable alternative that meets their needs. This approach has the support of healthcare professionals in the NHS and private sector, who recognise the importance of ensuring that patients have uninterrupted access to essential medications.
Our Role in the Creon Crisis
Smartway have been supplying Canadian Creon to a wide range of NHS pharmacies and hospitals since the start of the UK shortage in response to requests by healthcare professionals. Thanks to the commitment of our team and the strength of our supply chain network, we have helped thousands of patients with pancreatic insufficiency and remain focused on continuing our service and support.
Ensuring continuous access to medicines like Creon is yet another example of how we help patients obtain critical medicines. As a leading supplier of pharmaceuticals, our mission is to ensure that patients receive the medication they need, securely and promptly. By leveraging our direct relationships with manufacturers and suppliers, we can respond rapidly to medicine shortages and continuously source new medicines globally. This enables us to effectively manage supply issues as they arise.
Upholding Patient Safety Through Quality-Controlled Imports
While the import of Canadian Creon provides an immediate solution, patient safety through continued access remains a priority. Health authorities are closely monitoring the process to ensure that imported medications are high quality, effective and safe for use. Healthcare professionals will still regularly advise their patients to consult them about any changes to their medication regimen and promptly report any side effects or concerns.
At Smartway, we adhere to rigorous regulatory standards to ensure that imported medications meet the applicable standards of safety, quality and efficacy. We work closely with health authorities, manufacturers, suppliers, and healthcare professionals to maintain the high standards of quality and care. This helps ensure patients receive only safe and effective treatments that have passed through strict procedures.
We source medicines exclusively from authorised and trusted suppliers, including manufacturers. Our regulatory teams evaluate each supplier to ensure they meet the required standards before considering an import. Holding approvals, authorisations, and licences from regulatory bodies including the MHRA, Home Office and the GPhC, we are committed to delivering safe medicines that patients can trust. We are proud to be working with the NHS and Department of Health to meet the challenge that shortages bring.
Ensuring a consistent and reliable supply of safe medicines, especially during shortages, is at the core of what we do. Our teams are trained and committed to navigating complex international supply chains, with patient safety and medicine integrity remaining our top priority. - Amit Shah, Director of International Procurement
Moving Forward
As the UK continues its efforts to stabilise the supply of Creon capsules, this challenge underscores the importance of building a more resilient supply chain for essential medications and to help lift the burden on the NHS and healthcare professionals. Proactively engaging with manufacturers and diversifying sources of pharmaceutical supplies will be vital to mitigating the current and future shortages. Equally important is the ongoing communication between health authorities, healthcare professionals, and patients, ensuring that those affected are well-informed and supported throughout such challenges. Healthcare professionals can help, by giving early information on possible shortages so that imports can be considered.
The shortage of Creon capsules in the UK has undoubtedly posed significant difficulties for many patients, but the import of licensed Canadian Creon provides a much-needed solution. By ensuring patients can access the medications they require, the healthcare system can help alleviate some of the stress and uncertainty caused by this crisis. Looking ahead, strengthening the pharmaceutical supply chain and continuing to prioritise patient safety will be crucial in ensuring that no one is left without essential treatments in the future.